What do Patients & Staff say about Research?
Without patient participation clinical trials could not take place. Many patients who take part in trials benefit from obtaining successful new treatments before they are available and experience improved quality of life.
Patient Stories
To give you an idea of what it is like to take part in a trial, some of our patients who have participated, share their stories in the videos below.
Press the play button to start the video. Please make sure you have speakers connected and they are switched on to hear the audio.
Phillip's Story - EuroAspire Research Study
Jane's Story - Oncology Research Study
Lauren's Story - Reproductive Health Study
Robert's Story
Participant in Research Experience Survey (PRES)
PRES is an annual nationally standardised survey used to collect adults and children's views and experiences of participating in NIHR supported research.
Patients who participated in research studies at WWL received a number of questions regarding their experience of a research study. Charts below show the total number of responses received from patients 2024/2025.
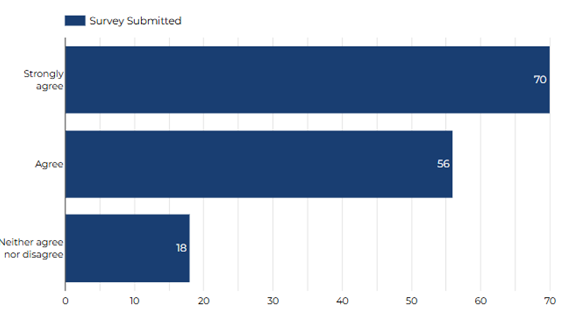
The information that I receive before taking part prepared me for my experience on the study.
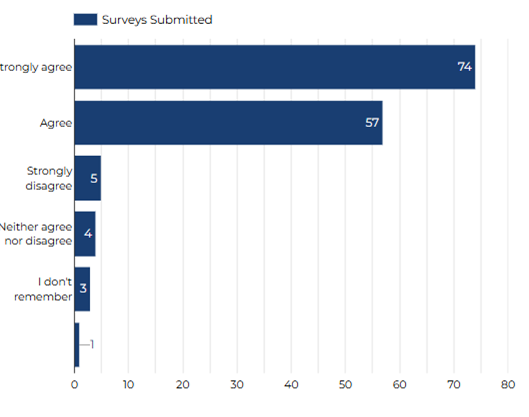
I feel research staff have valued my taking part in research study.
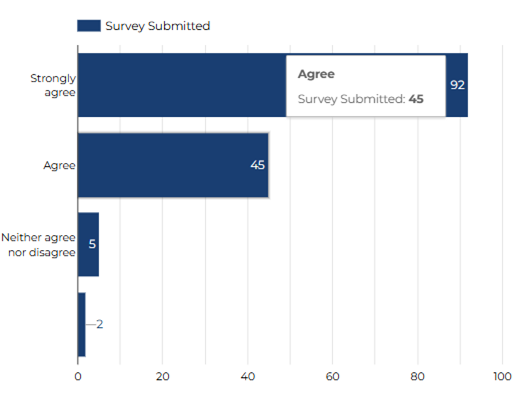
I would consider taking part in Research again.
For further information on PRES and PRES findings from 2021 thriy2024, please visit the NIHR PRES page