Performance in Research
Our Performance
WWL’s ambition is to align with the national focus from Government following the previously commissioned independent review led by Lord O’Shaughnessy, recommending how to resolve key challenges in conducting commercial clinical trials in the UK and transform the UK commercial clinical trial environment.
WWL is fully committed to provide more opportunities for our patients to have early access to innovative treatments that could improve, extend, or even save their lives.
Our Research Strategy ‘Research for All’ sets out a clear vision to expand the scope and reach of research and therefore, WWL welcomes the Government’s positive response to the Lord O’Shaughnessy review.
Our approach aligns with the NHS England published guidance around embedding research in the NHS, and how NHS organisations can manage research finance in the NHS (April 2024) to support building research capacity and capability.
To that end, we will ensure that commercial research we deliver achieves national Key Performance Measures which will be introduced across the NHS through the newly formed Research Delivery Network and in support of Industry-led trials in the UK.
As a minimum we will:
- Achieve Site Set-up Timelines (less than 60 days from receipt of full information pack).
- Achieve >80% Recruitment of participants ‘on Time’ and ‘to Target’ (RTT KPI)
- Re-invest commercial trials income into research infrastructure.
- Fully comply with national approaches to costing and contracting.
- Utilise all potential recruitment opportunities working with our health and care partners to reach under-served people.
WWL performance
In 2022/2023 WWL recruited 306 participants to commercial trials (4th highest in the Greater Manchester table for both numbers of studies and number of participants recruited).
2023/2024 annual figures published WWL recruited 61 participants to 8 commercial trials (12% of the portfolio).
Whilst the numbers of commercial trials delivered by WWL was relatively small, those which closed ‘in-year’ were delivered on Time and to Target (100% KPI).
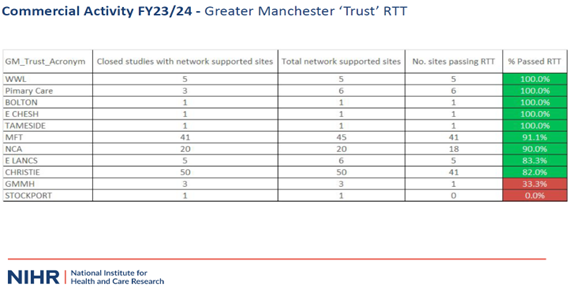
- Performance in Delivering Research
The Government's Plan for Growth, published in October 2022, announced the transformation of incentives at local level for efficiency in initiation and delivery of clinical research.
The National Institute of Health Research (NIHR) has therefore established 2 performance benchmarks that we and other NHS providers are measured against.
As a major UK Clinical Trials Unit with improvement in patient outcomes our primary aim, we are committed to the principle of initiating studies as efficiently as possible and delivering planned recruitment to time and target and therefore we have welcomed these benchmarks.
- Archive of Performance Indicators
- Initiating Clinical Research- the 70 day benchmark
Clinical trials to be set-up and operational within a 70 day period (basically this looks at how quickly studies are set-up and recruited to) - Delivering Clinical Research- time and target
Recruitment of commercial clinical trials to time and target (basically this looks at whether or not we've recruited the target number of patients in the timeframe we said we would). The overall aim here is to increase the number of patients who have the opportunity to participate in research and to enhance the nation's attractiveness as a host for research.
As a specialist rheumatology centre, we also host quite a lot of clinical trials in this disease , which sometimes means it often takes some time to identify/recruit the first patient and thus the 70-day benchmark is not feasible for some studies..
National Institute for Health Research Submission List - Link
Performance of Initiating Clinical Trials
Performance of Initiating Studies - Q4 19/20
Performance of Initiating Studies - Q1 20/21
Performance of Initiating Studies - Q2 20/21
Performance of Initiating Studies - Q3 20/21
Performance of Initiating Studies - Q4 20/21
Performance of Initiating Studies - Q1 21/22
Performance of Initiating Studies - Q2 21/22
Performance of Initiating Studies - Q3 21/22
Performance of Initiating Studies - Q4 21/22
Performance of Initiating Studies - Q1 22/23
Performance in Delivering
Performance in Delivering Studies - Q4 19/20
Performance in Delivering Studies - Q1 20/21
Performance in Delivering Studies - Q2 20/21
Performance in Delivering Studies - Q3 20/21
Performance in Delivering Studies - Q4 20/21
Performance in Delivering Studies - Q1 21/22
Performance in Delivering Studies - Q2 21/22
Performance in Delivering Studies - Q3 21/22
- Initiating Clinical Research- the 70 day benchmark